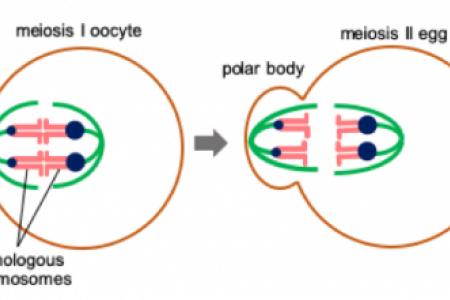
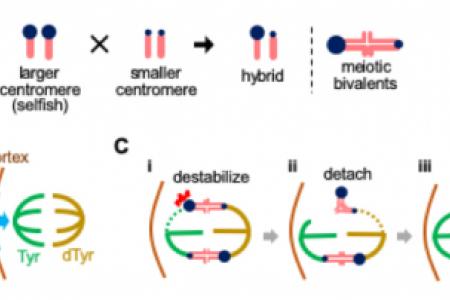
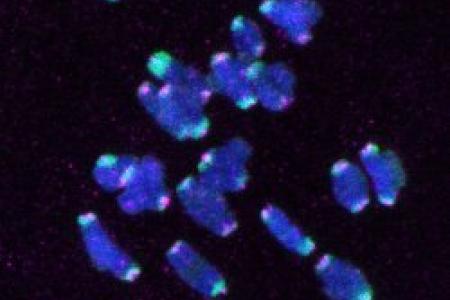
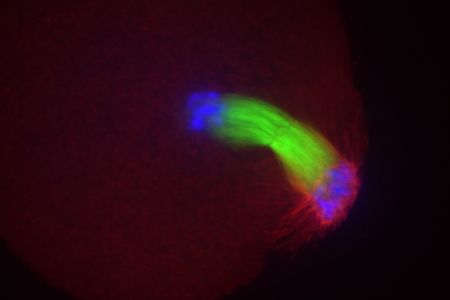
Meet the Team

Takashi Akera, Ph.D.
Dr. Takashi Akera graduated from the University of Tokyo with a Ph.D. in Biophysics and Biochemistry in 2014. He conducted postdoctoral research at the University of Pennsylvania in the laboratory of Dr. Michael Lampson from 2015 to 2019. He received both Holtzer Award for outstanding postdoctoral research in Cell and Developmental Biology and Kaushal Award for excellence in postdoctoral research in Genetics from the University of Pennsylvania in 2018. He was also a finalist for the ASCB Porter Prize for Research Excellence Award in 2018. Dr. Akera joined the NHLBI in 2019 as an Earl Stadtman tenure-track Investigator and is a member of the American Society for Cell Biology.
Contact the lab

Betsy Clark, PhD

Warif El Yakoubi, PhD

Eddie (Bo) Pan, PhD

Zaak Walton

Takaya Totsuka, Ph.D.

Duílio Silva, PhD

Sebastian Khan