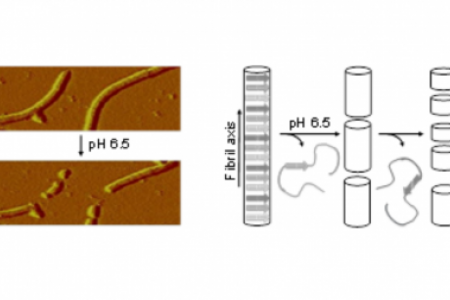
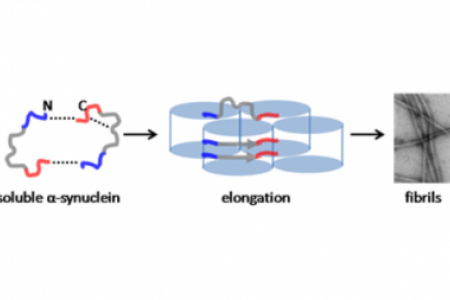
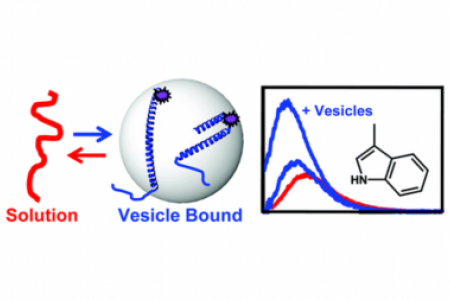
Meet the Team

Jennifer Lee, Ph.D.
Jennifer C. Lee graduated with a B.S. in chemistry and a B.A. in economics from the University of California, Berkeley, in 1997, and earned her Ph.D. in chemistry from the California Institute of Technology in 2002. Following a one-year postdoctoral stint at the University of Southern California, she became a Beckman Senior Research Fellow at the Beckman Institute Laser Resource Center at the California Institute of Technology where she investigated the structures and dynamics of an intrinsically disordered and amyloid forming protein using time-resolved spectroscopic measurements. In 2006, Dr. Lee joined the NHLBI as a tenure-track Investigtor. She was awarded an NIH Graduate Partnerships Program Outstanding Mentor Award in 2009. Dr. Lee is a member of the American Chemical Society and the Protein Society.
Contact the lab

Matthew Watson, Ph.D.

Ryan McGlinchey, Ph.D.

Sashary Ramos, Ph.D.

Daniel SanGiovanni

Tai Vu

Kevin Gery

Join Our Lab
Postdoctoral Fellowship
In expanding the scope of our research, a candidate with strong backgrounds in cell biology and fluorescence microscopy, including…